what must happen to the electrons in a material to create an electric current?
Imagine a world without batteries. All those portable devices nosotros're then dependent on would be so limited! We'd only be able to accept our laptops and phones as far as the reach of their cables, making that new running app you simply downloaded onto your telephone fairly useless.
Luckily, we practise take batteries. Back in 150 BC in Mesopotamia, the Parthian culture used a device known every bit the Baghdad battery, fabricated of copper and atomic number 26 electrodes with vinegar or citric acid. Archaeologists believe these were not really batteries simply were used primarily for religious ceremonies.
The invention of the battery equally we know information technology is credited to the Italian scientist Alessandro Volta, who put together the first battery to show a point to some other Italian scientist, Luigi Galvani. In 1780, Galvani had shown that the legs of frogs hanging on atomic number 26 or brass hooks would twitch when touched with a probe of some other blazon of metal. He believed that this was caused by electricity from within the frogs' tissues, and called it 'brute electricity'.
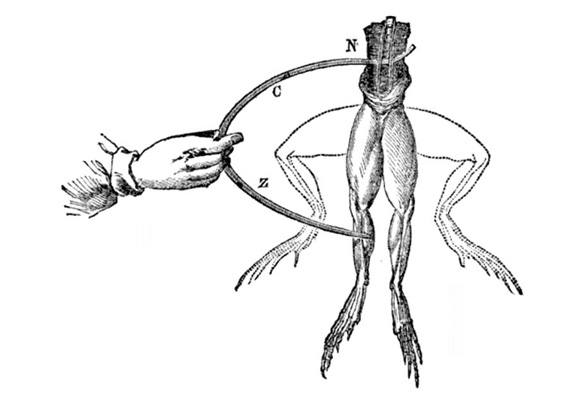
Volta, while initially impressed with Galvani'due south findings, came to believe that the electric electric current came from the two different types of metallic (the hooks on which the frogs were hanging and the different metal of the probe) and was merely being transmitted through, not from, the frogs' tissues. He experimented with stacks of layers of silver and zinc interspersed with layers of cloth or paper soaked in saltwater, and institute that an electric current did in fact flow through a wire applied to both ends of the pile.

Volta as well plant that past using different metals in the pile, the amount of voltage could be increased. He described his findings in a letter to Joseph Banks, then president of the Royal Society of London, in 1800. Information technology was a pretty big bargain (Napoleon was fairly impressed!) and his invention earned him sustained recognition in the honour of the 'volt' (a measure of electric potential) being named later him.
I myself, joking aside, am amazed how my old and new discoveries of ... pure and simple electricity caused by the contact of metals, could accept produced so much excitement.Alessandro Volta
So what exactly was happening with those layers of zinc and silver, and indeed, the twitching frogs' legs?
The chemistry of a bombardment
A bombardment is a device that stores chemical energy, and converts it to electricity. This is known as electrochemistry and the organisation that underpins a bombardment is called an electrochemical jail cell. A battery tin can be made up of one or several (like in Volta's original pile) electrochemical cells. Each electrochemical prison cell consists of ii electrodes separated by an electrolyte.
So where does an electrochemical cell get its electricity from? To answer this question, we demand to know what electricity is. Virtually merely, electricity is a type of free energy produced by the flow of electrons. In an electrochemical jail cell, electrons are produced past a chemical reaction that happens at one electrode (more than about electrodes below!) and and then they period over to the other electrode where they are used up. To understand this properly, we need to accept a closer look at the cell's components, and how they are put together.
Electrodes
To produce a period of electrons, you demand to have somewhere for the electrons to flow from, and somewhere for the electrons to catamenia to. These are the cell's electrodes. The electrons flow from ane electrode called the anode (or negative electrode) to another electrode called the cathode (the positive electrode). These are mostly different types of metals or other chemic compounds.
In Volta's pile, the anode was the zinc, from which electrons flowed through the wire (when connected) to the silver, which was the battery'due south cathode. He stacked lots of these cells together to make the total pile and crank up the voltage.
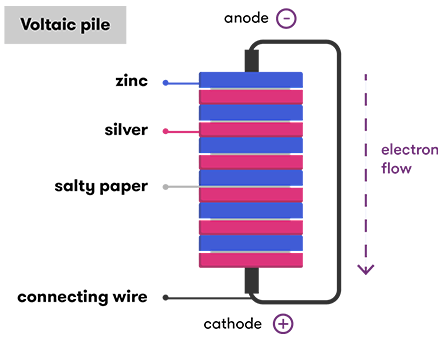
But where does the anode go all these electrons from in the first identify? And why are they and then happy to be sent off on their merry way over to the cathode? It all comes down to the chemistry that's going on inside the cell.
There are a couple of chemic reactions going on that we demand to understand. At the anode, the electrode reacts with the electrolyte in a reaction that produces electrons. These electrons accrue at the anode. Meanwhile, at the cathode, another chemical reaction occurs simultaneously that enables that electrode to accept electrons.
The technical chemical term for a reaction that involves the substitution of electrons is a reduction-oxidation reaction, more than commonly chosen a redox reaction. The entire reaction can be split into two half-reactions, and in the instance of an electrochemical cell, one half-reaction occurs at the anode, the other at the cathode. Reduction is the gain of electrons, and is what occurs at the cathode; nosotros say that the cathode is reduced during the reaction. Oxidation is the loss of electrons, so we say that the anode is oxidised.
Each of these reactions has a particular standard potential. Think of this feature as the reaction's ability/efficiency to either produce or suck upward electrons—its strength in an electron tug-of-war.
Any two conducting materials that take reactions with different standard potentials tin form an electrochemical prison cell, because the stronger one will be able to accept electrons from the weaker one. But the ideal pick for an anode would be a textile that produces a reaction with a significantly lower (more negative) standard potential than the material you lot choose for your cathode. What we end up with is electrons being attracted to the cathode from the anode (and the anode not trying to fight very much), and when provided with an easy pathway to get there—a conducting wire—we can harness their energy to provide electric power to our torch, phone, or whatsoever.
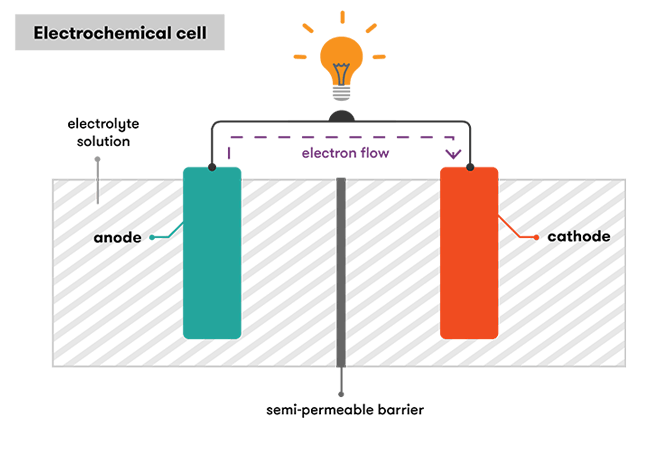
The difference in standard potential between the electrodes kind of equates to the force with which electrons will travel betwixt the two electrodes. This is known every bit the cell's overall electrochemical potential, and information technology determines the jail cell'south voltage. The greater the deviation, the greater the electrochemical potential, and the higher the voltage.
To increase a battery'due south voltage, nosotros've got ii options. We could choose different materials for our electrodes, ones that will give the cell a greater electrochemical potential. Or, we tin can stack several cells together. When the cells are combined in a particular way (in series), it has an additive effect on the battery'due south voltage. Essentially, the force at which the electrons move through the battery can be seen equally the total force every bit information technology moves from the anode of the first cell all the manner through even so many cells the battery contains to the cathode of the final cell.
When cells are combined in another fashion (in parallel) it increases the battery's possible current, which can be idea of every bit the total number of electrons flowing through the cells, only not its voltage.
Electrolyte
Only the electrodes are just role of the battery. Call up Volta's $.25 of paper soaked in salty h2o? The salty water was the electrolyte, another crucial part of the picture. An electrolyte can be a liquid, gel or a solid substance, but it must exist able to let the movement of charged ions.
Electrons have a negative accuse, and as nosotros're sending the catamenia of negative electrons effectually through our circuit, we need a way to residue that accuse movement. The electrolyte provides a medium through which charge-balancing positive ions can flow.
As the chemical reaction at the anode produces electrons, to maintain a neutral accuse balance on the electrode, a matching corporeality of positively charged ions are likewise produced. These don't get downwardly the external wire (that'southward for electrons only!) but are released into the electrolyte.
At the same time, the cathode must too remainder the negative charge of the electrons information technology receives, and then the reaction that occurs hither must pull in positively charged ions from the electrolyte (alternatively, it may also release negative charged ions from the electrode into the electrolyte).
Then, while the external wire provides the pathway for the period of negatively charged electrons, the electrolyte provides the pathway for the transfer of positively charged ions to balance the negative menses. This flow of positively charged ions is just every bit important as the electrons that provide the electric electric current in the external circuit we use to power our devices. The charge balancing role they perform is necessary to keep the entire reaction running.
Now, if all the ions released into the electrolyte were allowed to motion completely freely through the electrolyte, they would stop upwardly coating the surfaces of the electrodes and clog the whole system upward. So the jail cell generally has some sort of barrier to prevent this from happening.
When the battery is being used, we have a situation where there is a continuous catamenia of electrons (through the external excursion) and positively charged ions (through the electrolyte). If this continuous flow is halted—if the circuit is open, like when your torch is turned off—the flow of electrons is halted. The charges will pile/build up and the chemical reactions driving the battery will stop.
Every bit the battery is used, and the reactions at both electrodes chug along, new chemical products are made. These reaction products can create a kind of resistance that can prevent the reaction from continuing with the same efficiency. When this resistance becomes too peachy, the reaction slows down. The electron tug-of-state of war between the cathode and anode also loses its strength and the electrons stop flowing. The battery slowly goes flat.
Recharging a battery
Some common batteries are single employ only (known as principal or dispensable batteries). The trip the electrons take from the anode over to the cathode is one-way. Either their electrodes get depleted as they release their positive or negative ions into the electrolyte, or the build-upward of reaction products on the electrodes prevents the reaction from continuing, and information technology'due south washed and dusted. The bombardment ends upwardly in the bin (or hopefully the recycling, just that's a whole other Nova topic).
Only. The groovy matter about that flow of ions and electrons as it takes place in some types of batteries that accept advisable electrode materials, is that it can also go backwards, taking our battery back to its starting point and giving it a whole new lease on life. But as batteries transformed the manner we've been able to use various electrical devices, rechargeable batteries accept further transformed those devices' utility and lifespans.
When nosotros connect an almost apartment battery to an external electricity source, and ship energy back in to the battery, it reverses the chemical reaction that occurred during belch. This sends the positive ions released from the anode into the electrolyte back to the anode, and the electrons that the cathode took in also back to the anode. The return of both the positive ions and electrons dorsum into the anode primes the organisation so it'southward set to run once more: your battery is recharged.
The procedure isn't perfect, however. The replacement of the negative and positive ions from the electrolyte back on to the relevant electrode as the bombardment is recharged isn't equally corking or as nicely structured as the electrode was in the offset identify. Each charge cycle degrades the electrodes just a little scrap more, pregnant the battery loses operation over time, which is why even rechargeable batteries don't keep on working forever.
Over the grade of several charge and discharge cycles, the shape of the bombardment'due south crystals becomes less ordered. This is exacerbated when a battery is discharged/recharged at a high charge per unit—for instance, if y'all drive your electric car in big bursts of speed rather than steadily. High-charge per unit cycling leads to the crystal structure becoming more matted, with a less efficient battery as a consequence.
Retentivity outcome and cocky belch
The almost-but-not-quite completely reversible discharge and recharge reactions too contribute to something chosen the 'memory effect'. When you recharge some types of rechargeable batteries without sufficiently discharging them first, they 'remember' where they were up to in earlier discharge cycles and don't recharge properly.
In some cells, it is caused by the way the metal and the electrolyte react to form a salt (and the way that salt then dissolves again and metal is replaced on the electrodes when y'all recharge it). We want our cells to have nice, uniform, small crystals of salt coating a perfect metallic surface, but that's not what nosotros get in the real earth! The style some crystals grade is very complex, and the way some metals deposit during recharge is also surprisingly complex, which is why some bombardment types accept a bigger memory outcome than others. The imperfections mainly depend on the charge state of the battery to showtime with, the temperature, charge voltage and charging current. Over fourth dimension, the imperfections in one accuse cycle can cause the aforementioned in the next accuse cycle, and and then on, and our battery picks upwardly some bad memories. The retentivity effect is stiff for some types of cells, such as nickel-based batteries. Other types, similar lithium-ion, don't suffer from this trouble.
Some other aspect of rechargeable batteries is that the chemistry that makes them rechargeable besides ways they have a college trend towards self-belch. This is when internal reactions occur within the battery jail cell even when the electrodes are not connected via the external circuit. This results in the cell losing some of its chemic free energy over fourth dimension. A high self-discharge rate seriously limits the life of the bombardment—and makes them dice during storage.
The lithium-ion batteries in our mobile phones take a pretty good self-discharge rate of around 2–3 per cent per month, and our lead-acid auto batteries are besides pretty reasonable—they tend to lose four–vi per cent per month. Nickel-based batteries lose around 10–xv per cent of their accuse per month, which is not very practiced if you plan to store a torch for a whole flavour when you don't need it! A not-rechargeable alkaline metal battery simply loses around ii–iii per cent of its accuse per twelvemonth.
Voltage, electric current, power, chapters … what's the departure?
All these words basically describe the strength of a bombardment, right? Well, sort of. But they're all subtly different.
Voltage = strength at which the reaction driving the battery pushes electrons through the cell. This is also known as electrical potential, and depends on the difference in potential between the reactions that occur at each of the electrodes, that is, how strongly the cathode will pull the electrons (through the circuit) from the anode. The college the voltage, the more work the aforementioned number of electrons can do.
Current = the number of electrons that happen to be passing through any one point of a circuit at a given time. The college the current, the more work it can do at the same voltage. Inside the prison cell, you can also remember of electric current as the number of ions moving through the electrolyte, times the charge of those ions.
Power = voltage x current. The college the power, the quicker the charge per unit at which a battery can practice piece of work—this human relationship shows how voltage and current are both important for working out what a bombardment is suitable for.
Capacity = the ability of the battery as a role of time, which is used to describe the length of fourth dimension a battery will be able to power a device for. A high-capacity battery will exist able to go along going for a longer menstruum before going flat/running out of current. Some batteries have a deplorable little quirk—if y'all try and draw as well much from them besides quickly, the chemic reactions involved can't keep up and the capacity is less! And so, we always have to be careful when we talk about battery chapters and remember what the battery is going to be used for.
Another popular term is 'energy density'. This is the amount of energy a device tin can hold per unit of measurement volume, in other words, how much blindside you get for your buck in terms of power vs. size. With a battery, generally the higher the energy density the better, as it means the battery can be smaller and more meaty, which is e'er a plus when you need it to power something you lot want to go on in your pocket. It's even a plus for electric cars—the battery has to fit in the car somehow!
For some applications, such as storing electricity at a renewable power institute like a wind or solar subcontract, a loftier free energy density isn't and so much of a problem, as they volition well-nigh likely have ample space to store the batteries. The main goal for this use would be to simply store as much electricity as possible, as safely and cheaply as possible.
Why and then many types?
A range of materials (it used to be just metals) can be used as the electrodes in a battery. Over the years, many, many different combinations have been tried out, but there are just a few that have actually gone the altitude. But why use different combinations of metals anyway? If you've got a pair of metals that work well together as electrodes, why bother messing around with others?
Different materials have unlike electrochemical properties, and so they produce different results when you put them together in a bombardment jail cell. For example, some combinations will produce a loftier voltage, very chop-chop, simply then driblet off chop-chop, unable to sustain that voltage for long. This is skilful if yous need to produce, say, a sudden flash of calorie-free like a camera flash.
Other combinations will only produce a trickle of electric current, but they'll keep that trickle going for ages. Nosotros don't demand a huge amount of current to power a smoke detector, for example, merely we practise want our fume detectors to keep going for a long fourth dimension.
Some other reason to use different combinations of metals is that ofttimes two or more battery cells need to be stacked to obtain the required voltage, and it turns out that some electrode combinations stack together much more happily than other combinations. For instance, the lithium iron phosphate batteries (a type of lithium-ion battery) used in electric cars stack together to make high voltage systems (100 or fifty-fifty more volts), but you'd never practise that with those NiCad Walkman batteries that get hot!
Our different needs over time have led to the development of a huge array of battery types. To read more than about them, and what the future holds for battery ability, check out our other Nova topics.
sleemanthriasself.blogspot.com
Source: https://www.science.org.au/curious/technology-future/batteries

0 Response to "what must happen to the electrons in a material to create an electric current?"
Post a Comment